Another good article from India - highlighting the need to transition to electric vehicles and in particular the attributes of Faradion's Na-ion battery technology:
https://www.fairobserver.com/region/central_south_asia/india-electric-vehicles-sodium-ion-batteries-electric-cars-india-47938/
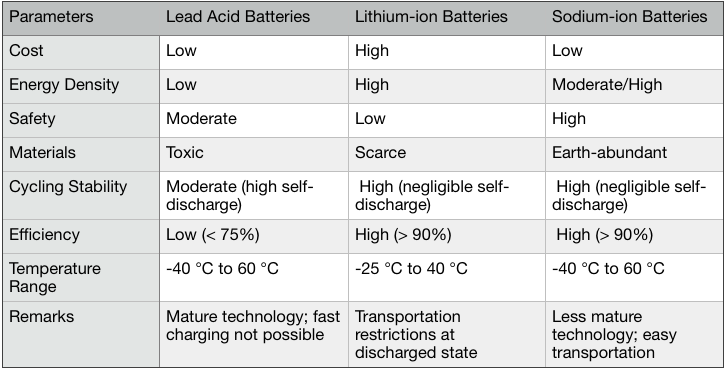
India should look at sodium–ion batteries more seriously. They work just like lithium–ion batteries, just with the lithium compounds swapped with sodium compounds. Sodium is one of the abundant elements on Earth, with vast global reserves of sodium minerals for example from seawater.
Research interest in sodium–ion batteries really took off from 2011. Until 2010, there were only 115 scientific papers ever published on such batteries by 2010. In the subsequent nine years, this number grew 50-fold to reach 5,804.
There are just a handful of companies working on this technology. The first to commercialize it, the UK-based Faradion, has filed multiple patents on sodium nickel and manganese-based oxide cathodes that do not contain any cobalt. Manganese and nickel are both abundantly available. The energy density of these sodium–ion cathodes has been shown to be almost similar (⁓80%) to that of NMC lithium–ion cathodes.
Sodium-ion batteries also have the significant advantages of being easier to recycle and transport. Lithium-ion batteries always need to be stored or transported at a partially or fully-charged state, where a battery is at its most unstable state. This is why there are global regulations that tightly dictate how lithium-ion batteries can be transported (why you can’t check-in such batteries in the luggage hold at the airport, for example).