Hi All,
Happy New Year to everyone!
I have been invited to give an oral presentation at the upcoming International Meeting on Lithium Batteries (IMLB 2020) in Berlin (June 2020).
https://www.imlb2020.org/
The IMLB is the premier international conference on the state of lithium (and sodium!) science and technology and is expected to draw more than 1000 delegates. It is intriguing that there is a special Na-ion session within the IMLB schedule - perhaps the organizers have seen the light!
The proposed title of my talk: The Commercialization of Safe, High Energy Density Na-ion Batteries.
I will have to demonstrate that Na-ion batteries are indeed the future! Safe, sustainable, outstanding performance plus zero lithium, zero cobalt and zero copper. What more do you need?
Here is the link to the list of invited speakers:
https://www.imlb2020.org/front/content.php?id_article=344
Jerry
Thursday, 2 January 2020
Thursday, 28 November 2019
India is at the Energy Crossroads - Time for Na-ion Batteries
Dear All,
Another good article from India - highlighting the need to transition to electric vehicles and in particular the attributes of Faradion's Na-ion battery technology:
https://www.fairobserver.com/region/central_south_asia/india-electric-vehicles-sodium-ion-batteries-electric-cars-india-47938/
Another good article from India - highlighting the need to transition to electric vehicles and in particular the attributes of Faradion's Na-ion battery technology:
https://www.fairobserver.com/region/central_south_asia/india-electric-vehicles-sodium-ion-batteries-electric-cars-india-47938/
India should look at sodium–ion batteries more seriously. They work just like lithium–ion batteries, just with the lithium compounds swapped with sodium compounds. Sodium is one of the abundant elements on Earth, with vast global reserves of sodium minerals for example from seawater.
Research interest in sodium–ion batteries really took off from 2011. Until 2010, there were only 115 scientific papers ever published on such batteries by 2010. In the subsequent nine years, this number grew 50-fold to reach 5,804.
There are just a handful of companies working on this technology. The first to commercialize it, the UK-based Faradion, has filed multiple patents on sodium nickel and manganese-based oxide cathodes that do not contain any cobalt. Manganese and nickel are both abundantly available. The energy density of these sodium–ion cathodes has been shown to be almost similar (⁓80%) to that of NMC lithium–ion cathodes.
Sodium-ion batteries also have the significant advantages of being easier to recycle and transport. Lithium-ion batteries always need to be stored or transported at a partially or fully-charged state, where a battery is at its most unstable state. This is why there are global regulations that tightly dictate how lithium-ion batteries can be transported (why you can’t check-in such batteries in the luggage hold at the airport, for example).
Friday, 15 November 2019
Can Sodium-ion Batteries Propel the Future of Clean Transportation?
Dear All,
A new article on Na-ion Batteries has been written by my colleague at Faradion, Dr. Ashish Rudola:
https://evreporter.com/sodium-ion-batteries/
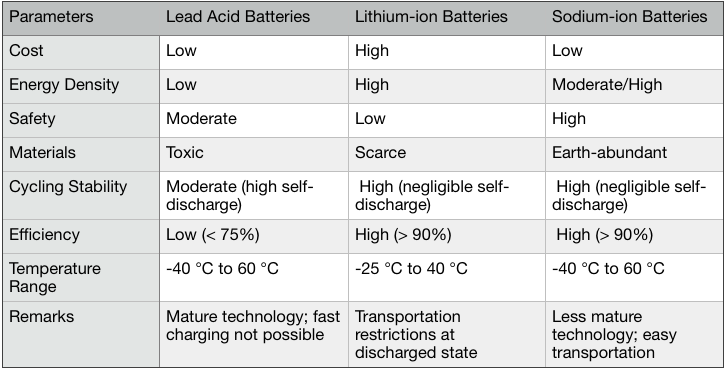
This level of technological maturity for sodium-ion batteries has been achieved in just eight years – with a few more years of similarly rapid development, the signs are indicating that the energy densities of commercial sodium-ion batteries would be comparable to those of NMC-based lithium-ion batteries. In the near future, it is realistic to expect that sodium-ion batteries would break through into the long-range EV market.
A new article on Na-ion Batteries has been written by my colleague at Faradion, Dr. Ashish Rudola:
https://evreporter.com/sodium-ion-batteries/

This level of technological maturity for sodium-ion batteries has been achieved in just eight years – with a few more years of similarly rapid development, the signs are indicating that the energy densities of commercial sodium-ion batteries would be comparable to those of NMC-based lithium-ion batteries. In the near future, it is realistic to expect that sodium-ion batteries would break through into the long-range EV market.
Thursday, 31 October 2019
Na-ion: A Battery Technology Worth Its Salt
Hi All,
Na-ion battery technology has been featured in an artice in Chemistry World, entitled A Battery Technology Worth Its Salt.

The full article may be found here:
https://www.chemistryworld.com/features/a-battery-technology-worth-its-salt/3010966.article
Na-ion battery technology has been featured in an artice in Chemistry World, entitled A Battery Technology Worth Its Salt.

The full article may be found here:
https://www.chemistryworld.com/features/a-battery-technology-worth-its-salt/3010966.article
Faradion, based in Sheffield, UK, was one of the first companies
on the sodium ion battery scene. ‘We have
a range of cathode materials patented, but our preferred composition right now
is a sodium–nickel layered oxide,’ explains Jerry Barker, chief
technology officer at Faradion. Graphite can’t be used as the anode in sodium
ion batteries; it is not energetically favourable to put sodium in between the
graphene layers. Faradion is using a hard carbon anode, a popular choice amongst
sodium ion battery developers, and NaPF6 electrolyte.
Alongside
batteries for renewable energy storage, Faradion is also developing replacements
for lead acid batteries in both low cost electric transport – such as e-bikes,
e-scooters and e-rickshaws – and for vehicle starter, lighting and ignition
(SLI) purposes. ‘We've done home storage system, e-bike and golf trolley
demonstrators to show that this technology is for real,’ says Barker. ‘We're
not anticipating manufacturing the batteries ourselves. We are relicensing the
technology now to existing lithium ion manufacturers and new entrants in the
field.’ He says they will hit the shelves: ‘hopefully, fairly soon ’.
Best, Jerry
Best, Jerry
Sunday, 27 October 2019
Faraday Institution - Expert Panel
Hi All,

I mentioned previously that I was acting as an Expert Panel member for the UK's Faraday Institution. The FI is the UK's independent institution for electrochemical energy storage and skills deveopment.
More information about the Faraday Institution and its mission may be found here:
https://faraday.ac.uk/#home
More information on the Expert Panel (including profiles) may be found here:
https://faraday.ac.uk/expert-panel/
Jerry

I mentioned previously that I was acting as an Expert Panel member for the UK's Faraday Institution. The FI is the UK's independent institution for electrochemical energy storage and skills deveopment.
More information about the Faraday Institution and its mission may be found here:
https://faraday.ac.uk/#home
More information on the Expert Panel (including profiles) may be found here:
https://faraday.ac.uk/expert-panel/
Jerry
Friday, 25 October 2019
International Conference on Sodium Batteries (ICNaB-2019)
Hiya,
International Conference on Sodium Batteries (ICNaB-2019).
The final program for the confernce in Naperville, Chicago is now avaiable here:
https://custom.cvent.com/39AB5C3D8FB74A4981E935F55018875E/files/f2388377bc3b411abbcb50e9b2d1c4ca.pdf
My abstract: The Performance and Commercialization of Faradion’s non-aqueous Na-ion Battery Technology Jerry Barker, Faradion Limited
Jerry
Thursday, 10 October 2019
Nobel Prize for Chemistry - 2019
Hi All,
I guess everyone is aware of this by now:
https://www.nobelprize.org/
The Nobel Prize in Chemistry 2019 has been awarded to John Goodenough, Stan Whittingham and Akira Yoshino. Richly deserved. The prize recognizes the development of the lithium-ion battery. This lightweight, rechargeable and powerful battery is now used in everything from mobile phones to laptops and electric vehicles. It can also store significant amounts of energy from solar and wind power, making possible a fossil fuel-free society.
To quote the Royal Swedish Academy of Sciences:
The foundation of the lithium-ion battery was laid during the oil crisis in the 1970s. Stanley Whittingham worked on developing methods that could lead to fossil fuel-free energy technologies. He started to research superconductors and discovered an extremely energy-rich material, which he used to create an innovative cathode in a lithium battery. This was made from titanium disulphide which, at a molecular level, has spaces that can house – intercalate – lithium ions.
I guess everyone is aware of this by now:
https://www.nobelprize.org/
The Nobel Prize in Chemistry 2019 has been awarded to John Goodenough, Stan Whittingham and Akira Yoshino. Richly deserved. The prize recognizes the development of the lithium-ion battery. This lightweight, rechargeable and powerful battery is now used in everything from mobile phones to laptops and electric vehicles. It can also store significant amounts of energy from solar and wind power, making possible a fossil fuel-free society.
To quote the Royal Swedish Academy of Sciences:
The foundation of the lithium-ion battery was laid during the oil crisis in the 1970s. Stanley Whittingham worked on developing methods that could lead to fossil fuel-free energy technologies. He started to research superconductors and discovered an extremely energy-rich material, which he used to create an innovative cathode in a lithium battery. This was made from titanium disulphide which, at a molecular level, has spaces that can house – intercalate – lithium ions.
The battery’s anode was partially made from metallic lithium, which has a strong drive to release electrons. This resulted in a battery that literally had great potential, just over two volts. However, metallic lithium is reactive and the battery was too explosive to be viable.
John Goodenough predicted that the cathode would have even greater potential if it was made using a metal oxide instead of a metal sulphide. After a systematic search, in 1980 he demonstrated that cobalt oxide with intercalated lithium ions can produce as much as four volts. This was an important breakthrough and would lead to much more powerful batteries.
With Goodenough’s cathode as a basis, Akira Yoshino created the first commercially viable lithium-ion battery in 1985. Rather than using reactive lithium in the anode, he used petroleum coke, a carbon material that, like the cathode’s cobalt oxide, can intercalate lithium ions.
The result was a lightweight, hardwearing battery that could be charged hundreds of times before its performance deteriorated. The advantage of lithium-ion batteries is that they are not based upon chemical reactions that break down the electrodes, but upon lithium ions flowing back and forth between the anode and cathode.
Monday, 7 October 2019
UK Faraday Institution
Hi All,
You are probably aware that the UK's Faraday Institution recently announced funding for 5 new research projects. That makes nine FI funded projects in total. At Faradion, we are particularly interested in the Sodium-ion Batteries project.
As you may know, I sit on the FI's Expert Panel:
https://faraday.ac.uk/expert-panel/
You are probably aware that the UK's Faraday Institution recently announced funding for 5 new research projects. That makes nine FI funded projects in total. At Faradion, we are particularly interested in the Sodium-ion Batteries project.
As you may know, I sit on the FI's Expert Panel:
https://faraday.ac.uk/expert-panel/
The Faraday Institution’s portfolio of large scale, mission-driven projects was selected after consultation with academic and industrial stakeholders across the country, with due consideration of the potential impact they could make to the UK. The institution’s four initial projects, launched in 2018 are as follows:
- • Extending battery life
- • Multi-scale modelling
- • Battery recycling and reuse
- • Solid state batteries
The following five further projects were launched in the second half of 2019.
- • Lithium ion cathode materials (2 projects)
Saturday, 5 October 2019
CEA Grenoble
Hi Everyone,
CEA-Grenoble. It was a great honour to be a member of the panel to examine Dr. Loic Simonin with a view to him being awarded the diploma HDR in Engineering - Materials. Loic did a great job!
CEA-Grenoble. It was a great honour to be a member of the panel to examine Dr. Loic Simonin with a view to him being awarded the diploma HDR in Engineering - Materials. Loic did a great job!
Also on the panel were my friends and colleagues: Laurence Croguennec (icmcb), Philippe Poizot (cnrs-imn), Rosa Palacin (icmab), Teofilo Rojo (cic-energigune) and Renaud Bouchet (grenoble-inp). As you can see, following the examination we had an excellent lunch!
Thursday, 5 September 2019
New Na-ion Battery - Wiki Page
Hiya Everyone,
My colleague at Faradion (with help from our friends at Tiamat Energy) has updated the Na-ion Battery Wikipedia page. This was long overdue as the last version contained a lot of errors and half-truths (and that is being polite!). It now looks really good. Take at look here:
https://en.wikipedia.org/wiki/Sodium-ion_battery
My colleague at Faradion (with help from our friends at Tiamat Energy) has updated the Na-ion Battery Wikipedia page. This was long overdue as the last version contained a lot of errors and half-truths (and that is being polite!). It now looks really good. Take at look here:
https://en.wikipedia.org/wiki/Sodium-ion_battery
Subscribe to:
Posts (Atom)


